Oxidation Number of Magnesium
In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero. While fully ionic bonds are not found in nature many bonds exhibit strong.
Oxidation State Examples Online Chemistry Tutor
Interestingly 2538 of people with type 2 diabetes are.

. Learn how to calculate or find the oxidation number of elements along with examples. Then you cover the Tickle with an argon blanket and react with hot magnesium at 850 degrees C to get the metallic element. Interactive periodic table with element scarcity SRI discovery dates melting and boiling points group block and period information.
2 ounces of almonds provide almost half that amount 150 mg of this important mineral. Therefore the valency of hydrogen is 1 as it can easily lose 1 electron and become stable. Oxidation State Number.
Covered in other articles. A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. After electrons were discovered chemists became convinced that oxidation-reduction reactions involved the transfer of electrons from one atom to another.
Write the oxidation number of each atom in the skeleton equation. Both hydrogen and oxygen have a possibility of 2 different oxidation. For example oxidation state of elemental atoms such as sodium magnesium iron is zero.
The genetic code is a set of three-nucleotide sets called codons and each three-nucleotide combination designates an amino acid for example AUG. Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of substrate change. That means the sulfur atom has.
Classically chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms with no change to the nuclei no change to the elements present and can often be described by a. Oxidation number also referred to as oxidation state is the number that is allocated to elements in a chemical combination. Thus everything that leads back to magnesium metal in the.
Found in all known forms of life ATP is often referred to as the molecular unit of currency of intracellular energy transfer. The oxidation state of. Similarly the net oxidation state of neutral.
The elements that are included in very few oxidation states are zinc and scandium. The reaction between magnesium oxide and carbon at 2000C to form magnesium metal and carbon monoxide is an example of the reduction of magnesium oxide to magnesium metal. Valency is different from the oxidation number and it has NO SIGN.
The current RDI for magnesium is 310420 mg. The 3xxx series comprises nearly 90 of all shaped. The positive oxidation state is the total number of electrons removed from the elemental state.
However some of the elements exhibit very few oxidation States. Editorauthors are masked to the peer review process and editorial decision-making of their own work and are not able to access this work. Bleaching something like hair blue jeans or glowing tonic water is also an oxidation-reduction.
And burning anything in oxygen for example sugar or magnesium is an oxidation reaction. The H ions with an oxidation number of 1 are reduced to H 2 with an oxidation number of 0 in the reaction. For example the oxidation of magnesium involves the chemical reaction between magnesium metal and oxygen to form magnesium oxide.
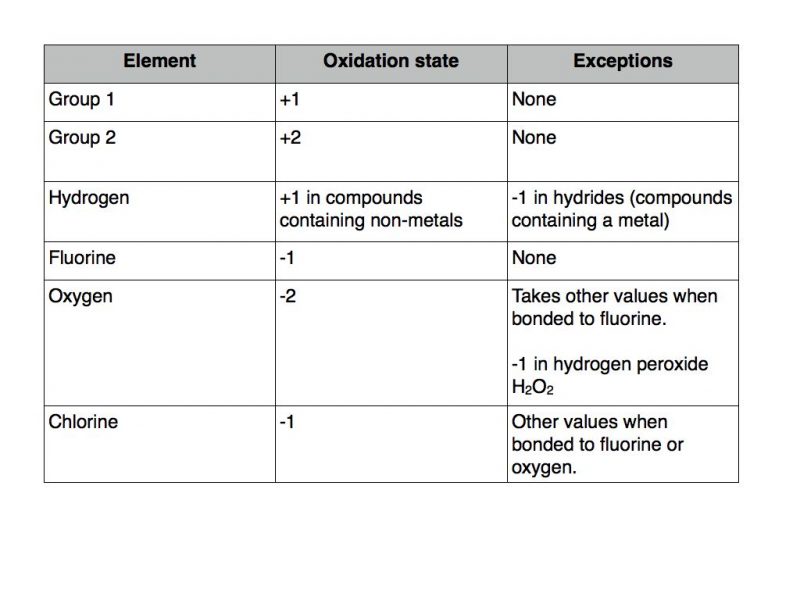
Group 2 metals in their elemental form such as magnesium and calcium have an oxidation number of 2. Oxidation number and oxidation state are two very important concepts in chemistry. Thus the valency of nitrogen.
If the ratio is equal to or slightly greater than unity an adherent non-porous protective oxide film forms such as typical on aluminium Fig. A number assigned to an atom describing its degree of oxidation meaning how many electrons it has gained or lost. When consumed in metabolic.
Have an oxidation number of 1. Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it. It is possible to remove a fifth electron to form another the ceVO_2 ion with the vanadium in a 5 oxidation state.
People call that Tickle. Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. Other alloying elements may be specified.
Country Most recent 10 notifications. A combination of complex liquids one suspended in the other serves as a factory for nanostructures with sought-after properties. Assign an oxidation number of 1 to hydrogen with exceptions.
It is -1 when combined with a metal. So the total oxidation number is -8. Adenosine triphosphate ATP is an organic compound that provides energy to drive many processes in living cells such as muscle contraction nerve impulse propagation condensate dissolution and chemical synthesis.
The ion on the whole has a -2 charge. Identifying Reduced and Oxidized Elements Determine which element is oxidized and which element is reduced in the following reactions be sure to include the oxidation state of each. On the other hand that of magnesium is 2 as it can lose 2 electrons easily and also attain stability.
European Union - 20220812 Draft COMMISSION IMPLEMENTING REGULATION EU laying down rules for the application of Regulation EU 2017745 of the European P View Notification View Notification. The oxidation state of magnesium has increased from 0 to 2. Like oxygen hydrogens oxidation number is subject to exceptional cases.
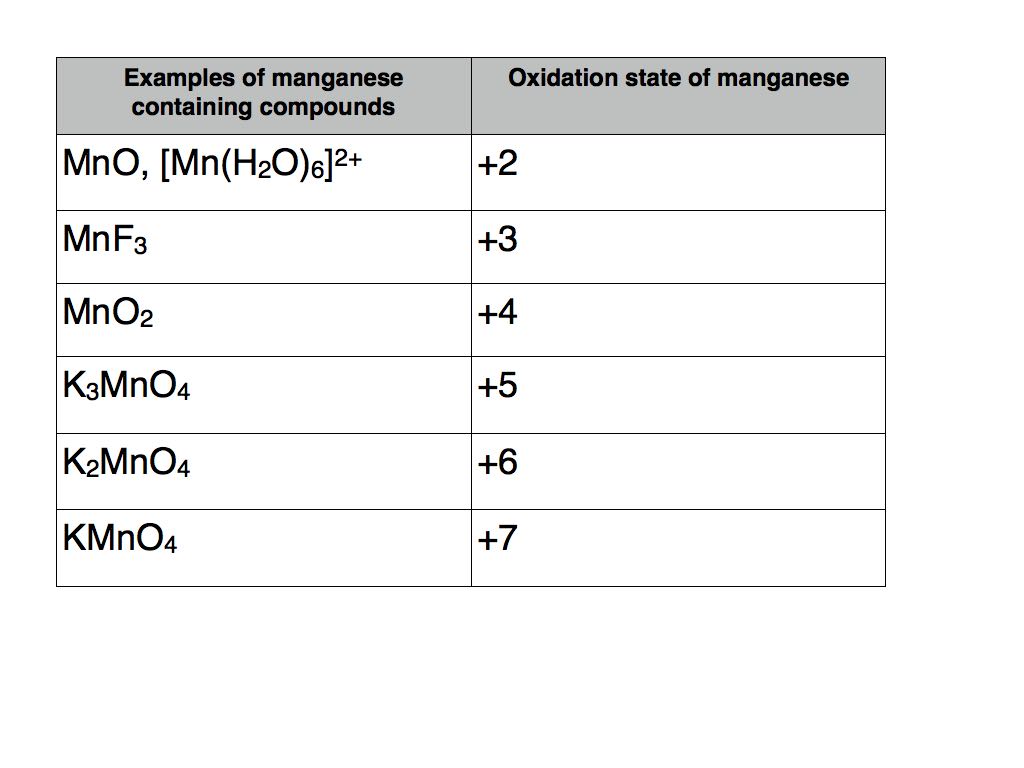
The other alloying elements such as copper and magnesium are specified. For example magnesium shows a wide range of oxidation states that starts from 2 and goes up to 7 in its various compounds. In this article we will make them very easy to understand.
The small number of oxidation states at the extreme left-hand side. Group 2 metals like calcium and magnesium are 2. Each protein has its own unique amino acid sequence that is specified by the nucleotide sequence of the gene encoding this protein.
Alloys in which silicon is the principal alloying element. The word reduction comes from the to lead back sense of the Latin stem. Oxidation continues rapidly as happens for a metal like magnesium Fig.
Hydrogen is 1 when combined with a non-metal for example hydrogen chloride HCL. European Union - 20220812 Draft COMMISSION IMPLEMENTING REGULATION EU. Proteins are assembled from amino acids using information encoded in genes.
Alloys in which copper is the principal alloying element. Zns 2H aq Zn 2 aq H 2 g Another simple example is the reaction between copper oxide and magnesium to yield copper and magnesium oxide. Difference between Valency and Oxidation Number.
Values are given for typical oxidation number and coordination. AJOGs Editors have active research programs and on occasion publish work in the Journal. The element has been oxidized.
Letting y be the oxidation number of phosphorus -1 y 21 4-2 y oxidation number of P 5. Controlled unalloyed pure compositions especially for rotor manufacture 2xxx. Generally speaking metals including sodium magnesium and iron are easily oxidized.
Reduction is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it.

Question Video Calculating Oxidation State Change For Magnesium During Magnesium Combustion Nagwa
Oxidation State Examples Online Chemistry Tutor

Oxidation Number State Definition Rules How To Find And Examples

No comments for "Oxidation Number of Magnesium"
Post a Comment